Biofilms and Oxygen
The inducible chemical-genetic fluorescent marker FAST outperforms classical fluorescent proteins in the quantitative reporting of bacterial biofilm dynamics
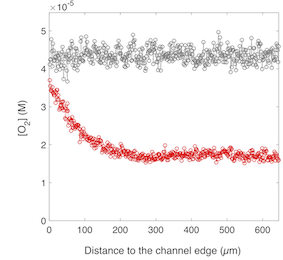
To increase our understanding of bacterial biofilm complexity, real- time quantitative analyses of the living community functions are required. To reach this goal, accurate fluorescent reporters are needed. In this paper, we used the classical fluorescent genetic reporters of the GFP family and demonstrated their limits in the context of a living biofilm. We showed that fluorescence signal saturated after only a few hours of growth and related this saturation to the reduction of oxygen concentration induced by bacterial consumption. This behaviour prevents the use of GFP-like fluorescent proteins for quantitative measurement in living biofilms. To overcome this limitation, we propose the use of a recently introduced small protein tag, FAST, which is fluorescent in the presence of an exogenously applied fluorogenic dye, enabling to avoid the oxygen sensitivity issue. We compared the ability of FAST to report on biofilm growth with that of GFP and mCherry, and demonstrated the superiority of the FAST:fluorogen probes for investigating dynamics in the complex environment of a living biofilm. (in coll. with Amaury Monmeyran, Philippe Thomen, Hugo Jonquière, Franck Sureau, Chenge Li, Marie-Aude Plamont, Jean-François Casella, Arnaud Gautier and Nelly Henry).
Bacterial biofilm under flow: First a physical struggle to stay, then a matter of breathing
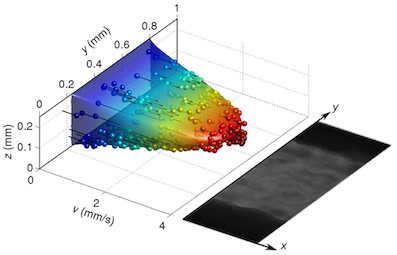
Bacterial communities attached to surfaces under fluid flow represent a widespread lifestyle of the microbial world. Through shear stress generation and molecular transport regulation, hydrodynamics conveys effects that are very different by nature but strongly coupled. To deci- pher the influence of these levers on bacterial biofilms immersed in moving fluids, we quantita- tively and simultaneously investigated physicochemical and biological properties of the biofilm. We designed a millifluidic setup allowing to control hydrodynamic conditions and to monitor biofilm development in real time using microscope imaging. We also conducted a transcrip- tomic analysis to detect a potential physiological response to hydrodynamics. We discovered that a threshold value of shear stress determined biofilm settlement, with sub-piconewton forces sufficient to prevent biofilm initiation. As a consequence, distinct hydrodynamic condi- tions, which set spatial distribution of shear stress, promoted distinct colonization patterns with consequences on the growth mode. However, no direct impact of mechanical forces on biofilm growth rate was observed. Consistently, no mechanosensing gene emerged from our differen- tial transcriptomic analysis comparing distinct hydrodynamic conditions. Instead, we found that hydrodynamic molecular transport crucially impacts biofilm growth by controlling oxygen availability. Our results shed light on biofilm response to hydrodynamics and open new ave- nues to achieve informed design of fluidic setups for investigating, engineering or fighting adherent communities. (in coll. with Philippe Thomen, Jérôme Robert, Amaury Monmeyran, Anne-Florence Bitbol and Nelly Henry).
An individual-based model for biofilm formation at liquid surfaces
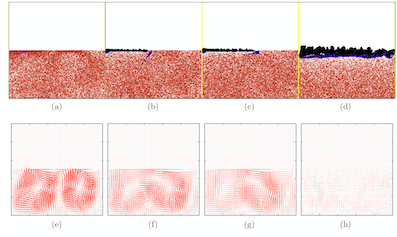
The bacterium Bacillus subtilis frequently forms biofilms at the interface between the culture medium and the air. We present a mathematical model that couples a description of bacteria as individual discrete objects to the standard advection-diffusion equations for the environment. The model takes into account two different bacterial phenotypes. In the motile state, bacteria swim and perform a run- and-tumble motion that is biased toward regions of high oxygen concentration (aerotaxis). In the matrix-producer state they excrete extracellular polymers, which allows them to connect to other bacteria and to form a biofilm. Bacteria are also advected by the fluid, and can trigger bioconvection. Numerical simulations of the model reproduce all the stages of biofilm formation observed in laboratory experiments. Finally, we study the influence of various model parameters on the dynamics and morphology of biofilms. (in coll. with Maxime Ardré, Hervé Henry and Mathis Plapp).