Mechanics of biofilms
La mécanique des biofilms à la surface de liquides
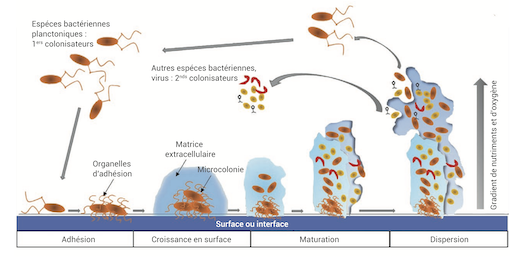
Les micro-organismes peuvent coloniser les surfaces environnantes et s’organiser en un film de plusieurs centaines de microns d’épaisseur, appelé « biofilm ». Ils sécrètent une matrice extracellulaire polymérique qui assure une véritable cohésion et protection physique de la colonie, avec des effets qui peuvent être aussi bien bénéfiques que mortels pour son environnement. Nous nous sommes intéressés aux forces mécaniques structurant cette matière vivante solide et contribuant à maintenir son intégrité. Nous avons travaillé sur des biofilms de bactéries flottant à la surface d’un liquide. Nous montrons que ces systèmes ont des propriétés mécaniques originales et remarquables, du fait de leur capacité à croître et proliférer. (in coll. with Virginie Bailleux, Catherine Even, Jean-Marc Allain, Christophe Regeard and Éric Raspaud).
Mechanical behavior of a Bacillus subtilis pellicle

Bacterial biofilms consist of a complex network of biopolymers embedded with microorganisms, and together these components form a physically robust structure that enables bacteria to grow in a protected environment. This structure can help unwanted biofilms persist in situations ranging from chronic infection to the biofouling of industrial equipment, but under certain circumstances it can allow the biofilm to disperse and colonize new niches. Mechanical properties are therefore a key aspect of biofilm life. In light of the recently discovered growth-induced compressive stress present within a biofilm, we studied the mechanical behavior of Bacillus subtilis pellicles, or biofilms at the air−liquid interface, and tracked simultaneously the force response and macroscopic structural changes during elongational deformations. We observed that pellicles behaved viscoelastically in response to small deformations, such that the growth-induced compressive stress was still present, and viscoplastically at large deformations, when the pellicles were under tension. In addition, by using particle imaging velocimetry we found that the pellicle deformations were nonaffine, indicating heterogeneous mechanical properties with the pellicle being more pliable near attachment surfaces. Overall, our results indicate that we must consider not only the viscoelastic but also the viscoplastic and mechanically heterogeneous nature of these structures to understand biofilm dispersal and removal. (in coll. with Emily C. Hollenbeck, Jean-Marc Allain, Philippe Roger, Christophe Regeard, Lynette Cegelski, Gerald G. Fuller and Éric Raspaud).
Bacillus subtilis bacteria generate an internal mechanical force within a biofilm
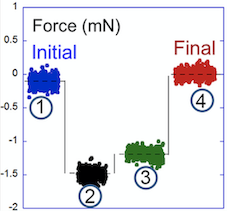
A key issue in understanding why biofilms are the most prevalent mode of bacterial life is the origin of the degree of resistance and protection that bacteria gain from self-organizing into biofilm communities. Our experiments suggest that their mechanical properties are a key factor. Experiments on pellicles, or floating biofilms, of Bacillus subtilis showed that while they are multiplying and secreting extracellular substances, bacteria create an internal force (associated with a !80 5 25 Pa stress) within the biofilms, similar to the forces that self-equilibrate and strengthen plants, organs, and some engineered buildings. Here, we found that this force, or stress, is associated with growth-induced pressure. Our observations indicate that due to such forces, biofilms spread after any cut or ablation by up to 15–20% of their initial size. The force relaxes over very short timescales (tens of milliseconds). We conclude that this force helps bacteria to shape the biofilm, improve its mechanical resistance, and facilitate its invasion and self-repair. (in coll. with Jean-Marc Allain and Éric Raspaud).
Elasticity and wrinkled morphology of Bacillus subtilis pellicles
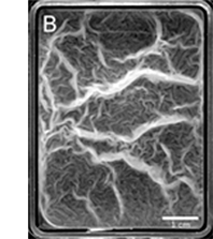
Wrinkled morphology is a distinctive phenotype observed in mature biofilms produced by a great number of bacteria. Here we study the formation of macroscopic structures (wrinkles and folds) observed during the maturation of Bacillus subtilis pellicles in relation to their mechanical response. We show how the mechanical buckling instability can explain their formation. By performing simple tests, we highlight the role of confining geometry and growth in determining the symmetry of wrinkles. We also experimentally demonstrate that the pellicles are soft elastic materials for small deformations induced by a tensile device. The wrinkled structures are then described by using the equations of elastic plates, which include the growth process as a simple parameter representing biomass production. This growth controls buckling instability, which triggers the formation of wrinkles. We also describe how the structure of ripples is modified when capillary effects are dominant. Finally, the experiments per- formed on a mutant strain indicate that the presence of an extracellular matrix is required to maintain a connective and elastic pellicle. (in coll. with Miguel Trejo, Virginie Bailleux, Christophe Poulard, Sandrine Mariot, Christophe Regeard and Éric Raspaud).