Baptiste Etcheverry, Marc baaden, Aurélien de la Lande, Fabien Cailliez, under review. Link to BioXxiv
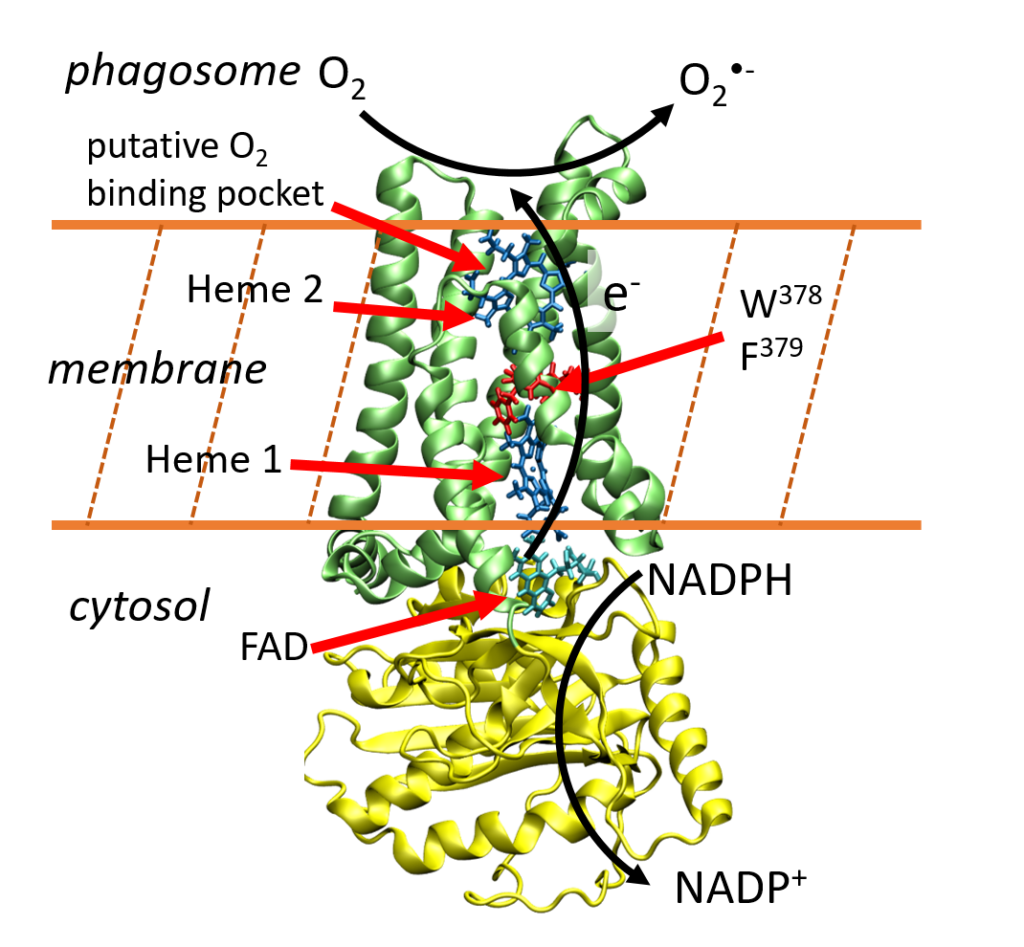
NADPH oxidases (NOX) form a family of transmembrane enzymes that catalyze the formation of reactive oxygen species. These are produced thanks to a chain of electron transfers (ET), shuttling electrons from one side of the membrane to the other, using one flavin and two heme cofactors as redox mediators. In this work we investigate the thermodynamics of the electron transfer (ET) between the two hemes contained in the transmembrane domain by means of extensive molecular dynamics simulations. We compare two proteins of the NOX5 isoform, from homo sapiens (hNOX5) and from cylindrospermum stagnale (csNOX5), a cyanobacteria. We study in detail the influence of both the density of negatively charged lipids in the membrane and of the NOX5 aminoacid sequence on the ET thermodynamic balance. The linear response formalism allows us to decompose the variation in free energy into the individual contributions of the system components (protein, membrane, solvent, etc.). We highlight the major compensatory effects of the various components in the global free energy budget in those complex systems. Although the contributions of the protein or the membrane to the ET thermodynamics can be individually strongly modified by a change in the aminoacid sequence or the membrane composition, they are largely compensated by the rest of the heme environment so that the total free energy is always found to be slightly favorable to the electron transfer. To our knowledge, this study is the first to highlight the effect of membrane charge density on inter-heme ET, providing valuable insights into the molecular mechanisms governing ET catalysis in complex membrane systems.